Abstract
Capsaicin is the molecule in peppers that produces a sensation of heat and pain when exposed to sensory nerve cells in the body. A series of ethylenediaminetetracetate bis-amide chelates have been synthesized with regions selective for capsaicinoid binding to determine which functional groups increase overall binding strength. The initial lanthanide complexes bound capsaicinoids with moderate strength (approximately 105 M-1). Additions to the bis-amide region of the chelating ligand aim to increase the selectivity of the lanthanide complex for capsaicinoids. Derivatives have been synthesized that contain aromatic rings and carbon chains to augment binding strength via the hydrophobic effect. Upon synthesis of the Eu3+ and Tb3+ complexes, luminescence spectroscopy is used to determine binding constants, binding stoichiometries, and the number of H2O molecules bonded to the metal ion in solution. The current synthesis, characterization and luminescence results for the complexes is reported.
Introduction
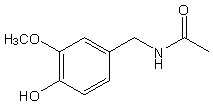
Capsaicin is part of a family of chemicals called vanilloids. It is naturally found in most types of chili and hot peppers. Capsaicin is biologically active and activates the sensory nerve fiber protein TRPV1 (transient receptor potential vanilloid subfamily 1)1. This nerve fiber is also activated by temperatures greater than 42°C. This is why when capsaicin activates this protein it creates the sensation of heat and pain, just like a burn, which is what causes the hot and spicy sensation one experiences when eating food prepared with peppers. Capsaicin is so pungent that 1/8000 mg can be detected with the human tongue by a distinct burning sensation2. It is also known that acidic conditions increase the sensitivity of the nerve fiber1. The structure of basic capsaicin is shown in figure 1. It is comprised of 3 main areas: the aromatic ring, a connecting polar group, and a hydrophobic chain1. Alteration to any of these 3 areas can drastically change the potency of the molecule. For example, if you remove the hydrophobic chain and replace it with a methyl group the pungency is effectively removed. Capsaicinoids have relevant use beyond the culinary world as well and also have medical applications as they contain many antimutagenic, antitumor, anti-inflammatory and antimicrobial properties, as well as being potent antioxidants and have been used as topical analgesics for pain3. Capsaicinoids work as pain relievers by desensitizing the applied area over time and are in use in several arthritis creams.
 Figure 1: Structure of Capsaicin
Figure 1: Structure of Capsaicin
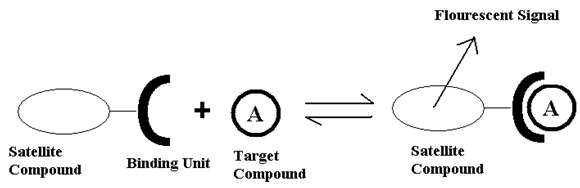
At this point detection methods for capsaicin analogs are highly limited to using high pressure liquid chromatography(HPLC). The goal of this project is to create an alternative way to detect capsaicin using luminescent lanthanide chelates in solution. Most trivalent lanthanides have luminescent states. Terbium (Tb3+) and europium (Eu3+) ions are luminescent in the green and red portions of the visible spectrum, respectively, and will be utilized in this study4. This type of detection is characterized by using a satellite compound that when bound to the lanthanide is able to excite the lanthanides’ 4f orbitals in a transition which gives a luminescent response as shown in figure 2. That luminescent response can be detected and used to determine the binding constant of the molecules in solutions of known concentrations with a fluorimeter. If the binding behavior is known it is possible to determine how much of the target compound was in solution4.
Figure 2: Example of Chemosensors5
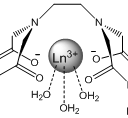
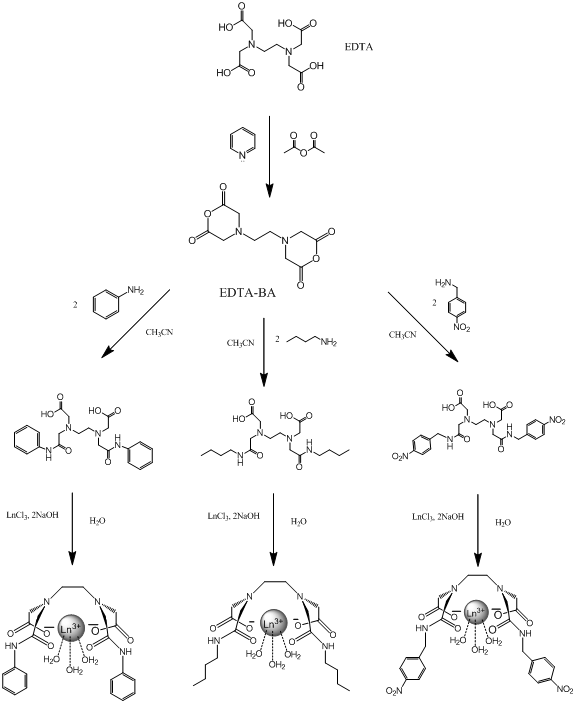
However, other molecules in a solution can react with the lanthanide as well and are able to affect the luminescent response by either quenching it or enhancing it, giving potentially false negatives and positives for the target compound6. Water is a well known quencher of lanthanide luminescence and will often be present in solutions7. One way to remove unwanted molecules is to create a chelate that binds to several of the lanthanides coordinating sites and is selective for the target compound. In this project analogs of EDTA(ethylenediaminetetracetate) are used due to its known strong complexation with many polyvalent metal ions, including lanthanides8,9. EDTA is a tetrabasic acid meaning that two hydrogens have strongly acidic properties, and the other two are weakly acidic9. Dr. Gulgas has already created an EDTA lanthanide complex shown in figure 3 that binds to capsaicin analogs with a luminescent response. However, the binding is weak to moderate(~1×105 M-1) 10.
Figure 3: Gulgas’ Chelate(R=CH3)
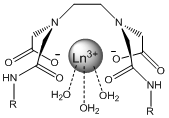
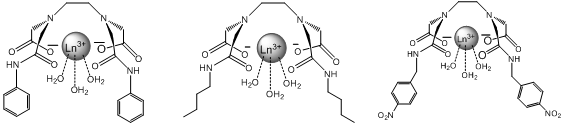
The main focus of this project will be altering the lanthanide complex to make it ideally suited as a highly sensitive and specific molecular probe for the capsaicin family. The approach to doing this will be the alteration to the R group bonded to the amide. These alterations include creating functional groups that are more similar to capsaicin, adding hydrophobic effects and creating functional groups that have the potential for more additions in the future. “Like molecules attract like molecules” and adding an aromatic group to the amide will make the chelate more selective for other aromatic molecules such as capsaicin. At the same time this addition could potentially drive away nonaromatic molecules such as H2O. However aromatic rings can also act as satellite compounds themselves due to their luminescent properties and could potentially give false positives in solution with capsaicin6. If the chelate is made more hydrophobic it will increase the binding strength because capsaicin is hydrophobic as well and they will be forced together in a mixture. For example, oil and water separate quickly when combined. If the chelate is made “oily” like the capsaicin then water (as the solvent) will drive them together. Figure 4 shows the alterations that will be discussed.
I have chosen these specific alterations because they make the complex more selective for capsaicin. The first alteration contains benzene rings similar to capsaicin and since “like molecules attract like molecules”, this will result in favorable interactions while potentially repelling other molecules in solution that do not contain benzene rings. The second alteration is for a similar reason. By adding a long carbon chain the complex becomes hydrophobic and will bind with capsaicin in solution, driven by the action of water molecules crowding the fatty chains together. The third alteration takes advantage of the aromatic properties as well as leaves the nitro group open to more additions in the future when the effects of different alterations is determined. For simplicity and safety reasons this project will use an analog of capsaicin called cap1 shown below.
Figure 5: Cap1
All chelates and metal complexes will be characterized with 1H-NMR and IR data. The binding constants will be determined by fluorescence titration. In general, solutions of target capsaicinoids will be titrated into a dilute solution of the synthesized lanthanide chelates, and the luminescence measured at each point to determine the binding stoichiometry and constant from the overall response profile. Instruments used will be a Varian Cary Eclipse Fluoresence Spectrophotometer, a Thermo Nicolet Smart MIRacle Avatar 360 FT-IR, and an Anasazi 60MHz NMR Spectrometer.
Experimental
Experiments start with synthesizing the bis-anhydride form of EDTA(EDTA-BA) which is a known molecule. This will be used as the starting material for all the alterations.
EDTA bis-phenylamide was synthesized by adding 4.56mL(50mmol) aniline dropwise to a round bottomed flask containing 2.988g(11.67mmol) EDTA-BA dissolved in about 20.0mL CH3CN. The solution turned light brown and warm, and was let stir for 2 days. A brown product formed and settled to bottom. The solution was filtered with a büchner funnel and rinsed with CH3CN and small amount of ether. A white powder filtered out and let dry. Yield: 4.22g. %Yield: 85.5%. 1H-NMR taken in D2O with Na2CO3.
The Tb3+ complex formed by dissolving the ligand in a 1:2 molar ratio of NaOH, and adding 1 equivalent of TbCl3 dissolved in MeOH and H2O. Let stir overnight. Product was filtered using a Hirsch funnel, white powder product let dry.
EDTA bis-butylamide was synthesized by adding 1.706mL(16.8mmol) butylamine dropwise to a round bottom flask containing 1.24g(4.00mmol) EDTA-BA dissolved in 7mL CH3CN. The solution turned gold and warm and was let stir for 2 days. No product came out of solution. The solution was evaporated for 2 hours and formed a golden solid around bottom of flask. The residue was treated with ether and a white solid precipitated out. Initial 1H-NMR shows excess butylamine. A silica column was ran with 10% methanol, 90% CH2Cl2 to separate product from excess reactants. Ninhydrin was used to detect amine presence. 1H-NMR still shows excess butylamine. Combined vials and evaporated to remove as much solvent as possible to form Ln3+ complexes. Yield: 0.1487g. %Yield: 7.63%. All 1H-NMR taken in D2O and NaCO3.
The Tb3+ complex formed by dissolving the ligand in a 1:2 molar ratio of NaOH, and adding 1 equivalent of TbCl3 dissolved in MeOH and H2O. Let stir overnight. No particles in solution. Evaporated for 30mins and dissolved in MeOH and precipitated out with ether. Product was white and cloudy.
EDTA bis-p-nitrobenzylamide was synthesized by adding 0.456g(3mmol) p-nitrobenzyl amine to a round bottomed flask containing 0.256g(1mmol) EDTA-BA dissolved in 3mL CH3CN. The solution turned dark yellow and was let stir overnight. Dissolved yellowish product in CH3Cl2 and sonicated. Product fell out of solution and was filtered in Hirsch funnel. Yield: .511g. %Yield: 91.3%. 1H-NMR taken in D2O and Na2CO3.
The Tb3+ complex formed by dissolving the ligand in a 1:2 molar ratio of NaOH, and adding 1 equivalent of TbCl3 dissolved in MeOH and H2O. Solution turned red briefly with addition of TbCl3. Filtered product in Hirsch funnel.
The titration experiment was carried out with 5×10-5M of the Tb3+ complexes starting with 0 equivalents of cap1. Scanned emission at 292nm and 315nm excitations, and excitation scans were performed at 615nm emission. Equivalents ranged from 0-2eq by 0.2 eq and 2-5eq by 1 eq and 5-35eq by 5eq.
Results and Discussion
Characterization
Characterization of the ligands was confirmed using 1H-NMR. Spectra shown below for all compounds and hydrogen groups labeled and identified.
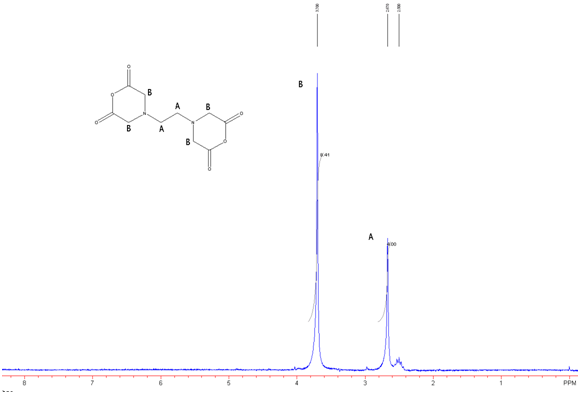
Figure 7: EDTA-BA in DMSO
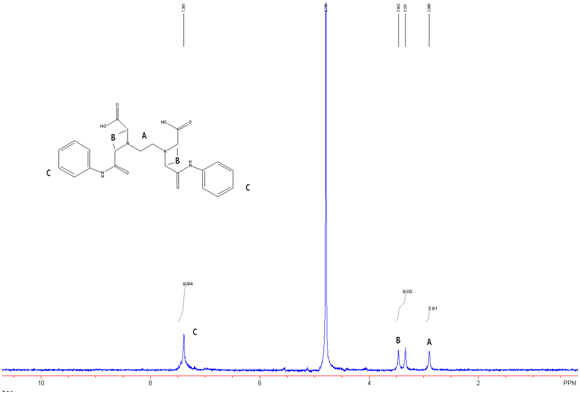
Figure 8: EDTA-bis-phenylamide in D2O w/ Na2CO3
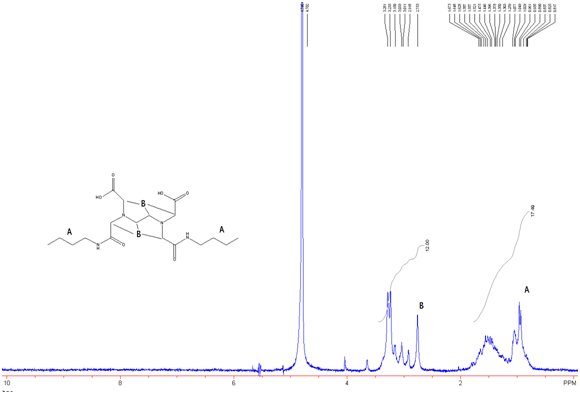
Figure 9: EDTA-bis-butylamide in D2O w/ Na2CO3
It is expected that there is excess butylamine in this product which is what leads to the integration values seen in figure 9.
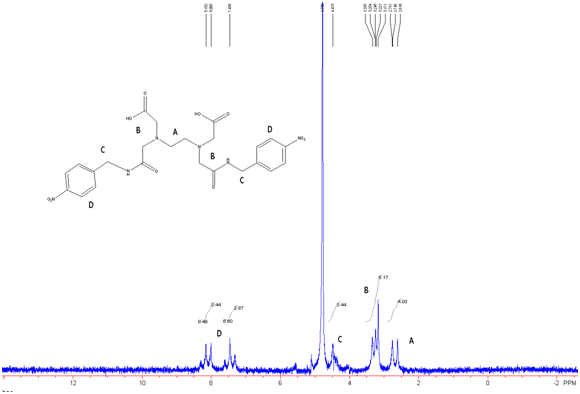
Figure 10: EDTA-bis-p-nitrobenzylamide in D2O w/ Na2CO3
IR spectra where taken of the compounds that formed Ln3+ complexes for proof that the lanthanide had bound to the chelates. If the lanthanide is bound then a shift to a lower wavenumber in the spectrum will be apparent.
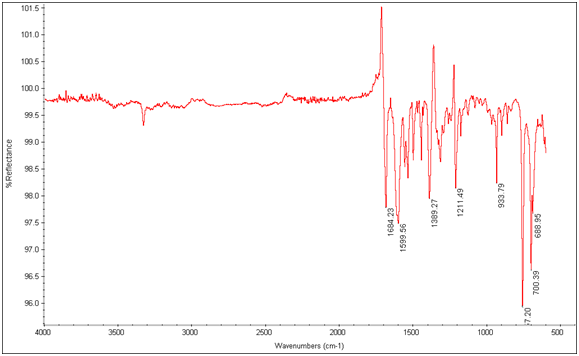 Figure 11: EDTA-bis-phenylamide
Figure 11: EDTA-bis-phenylamide
Figure 12: EDTA-bis-phenylamide Tb3+ complex
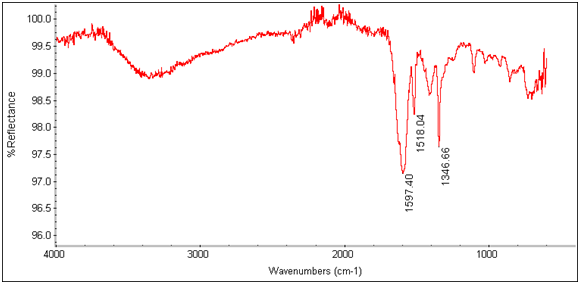
There is an evident shift in the spectrum from 1684cm-1 to 1652cm-1 in the expected Tb3+ complex trial, indicating that the lanthanide has bonded to the chelate and is affecting its IR absorbtion. This is also seen in the EDTA-bis-p-nitrobenzylamide chelate and Tb3+ complex’s spectra as shown below.
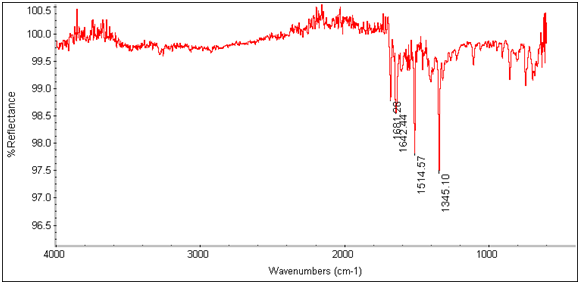 Figure 13: EDTA-bis-p-nitrobenzylamide
Figure 13: EDTA-bis-p-nitrobenzylamide
Figure 14: EDTA-bis-p-nitrobenzylamide Tb3+ Complex
IR data from the EDTA-bis-butylamide chelate and complex not available due to excess of butylamine in product and failure to complex with Tb3+ successfully.
Luminescence
Characterization of the EDTA-bis-phenylamide and EDTA-bis-p-nitrobenzylamide were a success and luminescence titrations were performed to determine whether the chelates are responsive to capsaicin(cap1). Figure 15 shows the emission spectrum for the original chelate complex this project was designed around and can be used as a reference to see how well the new additions affect the luminescence.
Figure 15: Gulgas’s Origional Chelate
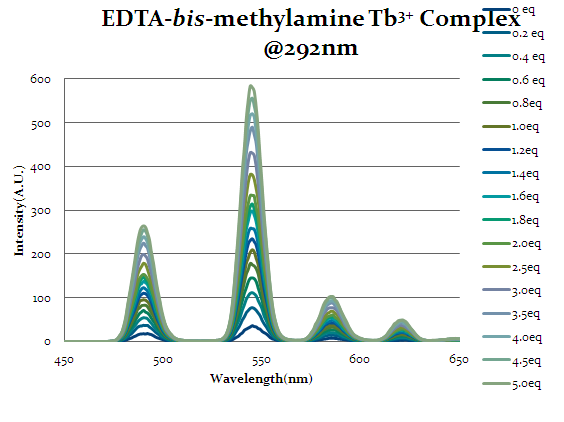
Intensity increases as more equivalents of cap1 are added which indicate that the chelate is binding to the cap1 and allowing cap1 to act as an antenna to excite the lanthanide.
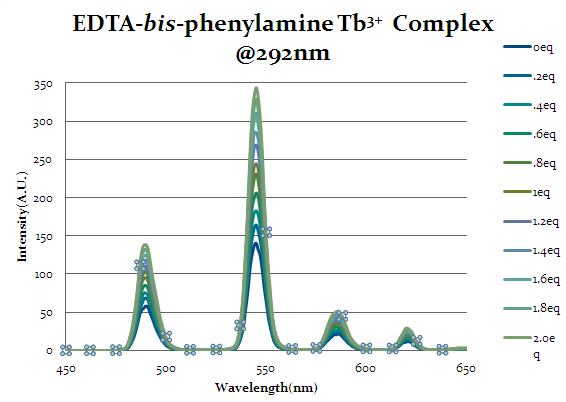
Figure 16
Seen above in figure 16 is the emission spectrum of EDTA-bis-phenylamide. Initial augmentation of the intensity prior to any equivalents of cap1 being added was expected due to the aromatic rings’ ability to act as an antenna group itself and excite the lanthanide. However, even with the chelate’s antenna capabilities an increase in intensity is seen as equivalents of cap1 are added indicates that the cap1 is binding to the complex with at least the same strength as the original chelate.
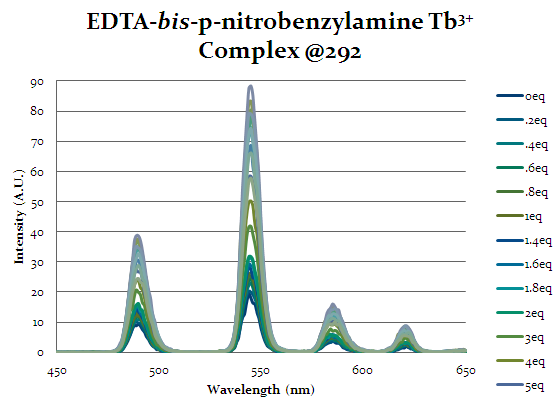
Figure 17 shows the emission spectra for EDTA-bis-p-nitrobenzylamide complex. This spectra shows the same increasing trend as the previous complexes indicating that cap1 is binding to the complex and exciting the lanthanide. This complex does not have as strong as a response as the other complexes indicating that it is not as strong of a selector for cap1. However, the purpose of this alteration was to allow for more additions in the future off the aromatic ring, so it’s ability to allow cap1 to bind is desirable, even if it is weaker than other complexes.
The excitation spectra of the complexes shows the same increasing trends, verifying the data from the emission spectra. Complexes with Eu3+ were also synthesized, but the luminescence data was not as intense for those complexes and is not shown here.
Conclusion and Future Work
While the binding constants have not yet been determined it is evident that two of the proposed chelate designs, the phenylamide and nitrobenzylamide, have had positive reactions with the target compound cap1. 1H-NMR and IR data have proven that both of these compounds have been able to be synthesized as predicted and are able to complex successfully with Tb3+ in solution. While the phenylamide showed a better response for cap1 in titration experiments, the nitrobenzylamide still maintains a response while allowing for future additions. Possibilities include trying to add a carbon chain just like the butylamide that was attemped in this project. It is possible that adding the chain farther away from the chelate off the benzyl ring might make for easier purification and successfull complexation. Other electron rich functional groups such as thioureas or carbonyl groups might also be utilized to try and hydrogen bond to the capsaicins’ connecting polar group. It is also possible to create a macrocyclic chelate that binds to the lanthanide more strongly to help inhibit H2O from coordinating.
Acknowledgements
I would like to acknowledge and thank those who have made it possible for me to carry out this research. I especially thank Dr. Gulgas for his guidance in this project and time invested. I thank L. Wyatt Colvin for his assistance with the luminescence experiments. I thank Longwood University for the instrumentation, funding, and facilities. I also thank those on my committee who will take the time out of their schedules to read this.
References
1. Rusterholz, David. “Capsaicin, from Hot to Not; Can New Pain-Relieving Drugs Be Derived from This Substance known To Cause Pain?.” Journal of Chemical Education. 83.12 (2006): 1809-1811
2. Nelson, E. K.. “Vanillyl-Acyl Amides.” The Essential Oils Laboratory, Drug Division, Bureau of Chemistry, U. S. Department of Agriculture. (1919): 2128
3. Barbero, G., J. Molinillo, R. Varela, M. Palma, and F. Macias. “Application of Hansch’s Model to Capsaicinoids and Capsinoids: A Study Using the Quantitative Structure-Activity Relationship. A Novel Method for the Synthesis of Capsinoids.” Agricultural and Food Chemistry. 58. (2010): 3342
4. Moore, E., Samuel, A., Raymond, K. “From Antenna to Assay: Lessons Learned in Lanthanide Luminescence.” Accounts of Chemical Research. 42. 4 (2009): 544-546
5. Martinez-Manez, R., and F. Sancenon. “Fluorgenic and Chromogenic Chromosensors and Reagents for Anions.” Chemical Reviews. 102.11 (2003): 4420
6. Cable, M., J. Kirby, K. Sorasaenee, H. Gray, A. Ponce. “Bacterial Spore Detection by [Tb3+ (macrocycle)(dipicolinate)] Luminescence.” Journal of the American Chemical Society. 129. 6 (2007): 1474
7. Cable, M., J. Kirby, D. Levine, M. Manary, H. Gray, A. Ponce. “Detection of Bacterial Spores with Lanthanide-Macrocycle Binary Complexes.” Journal of the American Chemical Society. 131. 27 (2009): 956
8. Michels, J., J. Huskens, D. Reinhoudt. “Noncovalent Binding of Sensitizers for Lanthanide(III) Luminescence in an EDTA-bis(β-cyclodextrin) Ligand” Journal of the American Chemcial Society. 124. 9 (2002): 2057
9. Johnston, M., A. Barnard, H. Flaschka. ”EDTA and Complex Formation.” Journal of Chemical Education. 35. 12(1958): 602
10. Gulgas, Christopher. Personal Interview. 20 Jul 2010