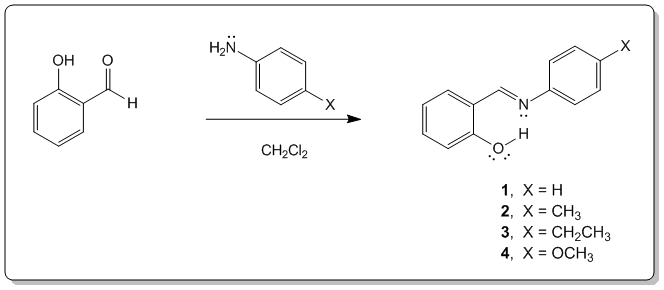
Abstract
The syntheses of salicylidene anilines are readily accomplished through one-pot procedures with minimal purification necessary to afford a functional crystalline product. These compounds are of interest due to their colorimetric response to 405 nm light in the solid state. A series of salicylidene anilines have been generated to study the effect of enol/trans-keto isomerization on the UV-Vis absorption behavior. The compounds are characterized using 1H-NMR and UV-Vis spectroscopies. A screening process based on colorimetric response, synthetic ease, and safety concerns has been applied to identify the most suitable candidates for illustrating important concepts in the high school chemistry curriculum. The synthesis and characterized response profile of the molecules and specific learning objectives for integration into a functional teaching unit shall be discussed.
Introduction
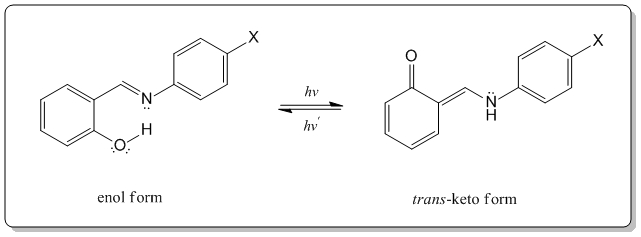
Salicylideneanilines belong to a class of organic compounds best known for exhibiting photochromism in the solid state. T hese crystals have been studied since 1962 for this unique property.1 Photochromism occurs when a substance absorbs light and changes color. Upon irradiation with near ultraviolet light (405nm) the observed color of the salicylideneanilines in this study deepens to red; when left in the dark or irradiated with visible light, they revert to their original color.2 This property is due to the isomerization between the enol and trans-keto forms of the molecule. In this transformation, the proton bonded to the oxygen atom is translocated to the imine nitrogen atom. 2
In order to view these properties salicylaldehyde was reacted with several anilines. Four of these molecules will be discussed, shown in Figure 1. Two are pale yellow crystals that are not fluorescent at room temperature and exhibit photochromism (molecule 1 and 4), while the other two (molecule 2 and 3) are not photochromic, but exhibit fluorescence in the solid state. This photochromic property can further be explained by the nonplanar nature of the molecules in the crystals that exhibit photochromism, therefore allowing a substantial twist in the N-aryl bond.2 Figure 2 illustrates the structural change that occurs during this isomerization.
These molecules are of interest in a high school lab setting due to their colorimetric response to 405nm light. Using an inexpensive blue laser pointer, students would be able to manipulate the crystals as well as observe how structural differences in a series of anilines affect the photochromism in the crystalline product. Also, since these crystals are easily synthesized with minimal purification necessary to afford a functional crystalline product, they are suitable candidates for an introduction into complicated organic structures and light absorption in a high school curriculum.
Methods
It is important to note that anilines are known to have some level of toxicity. Manipulation of these solids should occur in a fume hood with adequate personal protective gear (gloves, goggles).
Molecule 1. Salicylaldehyde (3.096g) and dichloromethane (DCM)(25ml) were added to a 250ml Erlenmeyer flask on a stir plate. Aniline (2.37g) was placed in a flask and dissolved with DCM (10ml). The diluted aniline was then pipetted drip by drip into the Erlenmeyer flask still on the stir plate; the solution immediately changed color. The solution was allowed to mix for 24 hours then left to crystallize. After a week, yellow crystals were created that were then crushed into a powder that became an orange color. This molecule showed photochromism. 1 H-NMR (CDCl3, 60 MHz) 7.06-7.40 (9H) 8.57 (1H) 13.4 (1H).
Molecule 2. Salicylaldehyde (.322g) and DCM (2.5ml) were added to a 25ml flask on a stir plate. P-Toluidine (.282g) was placed in a flask and dissolved with DCM (1ml). The diluted aniline was then pipetted drip by drip into the Erlenmeyer flask still on the stir plate. The solution was allowed to mix for 24 hours then left to crystallize. After a week the stir bar was removed and then the solution began to crystallize. The molecule was then recrystallized by adding boiling methyl alcohol to product on hot plate until completely dissolved. Once dissolved solution was removed from heat and a watch plate was placed on top of the flask to allow slow recrystallization. When solvent was almost completely evaporated solution was vacuum filtered to obtain pure crystals. Molecule showed florescence but not photochromism. 1H-NMR(CDCl3, 60 MHz) 2.37 (3H) 7.03-7.37 (8H) 8.60 (1H) 13.34 (1H).
Molecule 3. Salicylaldehyde (.306g) and DCM (2.5ml) were added to a 25ml flask on a stir plate. 4-Ethylaniline (.303g) was placed in a flask and dissolved with DCM (1ml). The diluted aniline was then pipetted drip by drip into the Erlenmeyer flask still on the stir plate. The solution was allowed to mix for 24 hours then left to crystallize. After a week the solution crystallized. The molecule was then recrystallized by adding boiling methyl alcohol to product on hot plate until completely dissolved. Once dissolved solution was removed from heat and a watch plate was placed on top of the flask to allow slow recrystallization. When solvent was almost completely evaporated solution was vacuum filtered to obtain pure crystals. Molecule showed florescence, but not photochromism. 1H-NMR(CDCl3, 60 MHz) 1.23 (s,3H) 2.72 (s, 2H) 7.01-7.35 (8H) 8.56 (1H) 13.33 (1H).
Molecule 4 Salicylaldehyde(3.28g) and DCM (25ml) were added to a 250ml flask on a stir plate. P-anisidine (3.31g) was placed in a flask and dissolved with DCM (10ml). The diluted aniline was then pipetted drip by drip into the Erlenmeyer flask still on the stir plate. The solution was allowed to mix for 24 hours, then left to crystallize. After a week the solution did not crystallize, therefore it was evaporated with N2 and vacuum filtered. Adding boiling methyl alcohol to product on hot plate until completely dissolved then recrystallized molecule. Once dissolved solution was removed from heat and a watch plate was placed on top of the flask to allow slow recrystallization. When solvent was almost completely evaporated solution was vacuum filtered to obtain pure crystals. Molecule showed photochromism, however the effect did not last as long as with molecule 1. 1H-NMR(CDCl3, 60 MHz) 1.34 (1H) 3.8 (2H) 7.02-7.26 (8H) 8.61 (1H) 13.3 (1H).
Results and Discussion
Molecule 1 was found to be photochromic after the simple synthesis. The product crystallized out and showed the desired property without recrystallization. Upon absorbing blue-violet light (405 nm) from a commercially available laser pointer, a color change from yellow-orange to deep red was observed on a solid sample of this molecule. The color change lasted several minutes and it was possible to draw a complex picture with the laser pointer. Figure 3 depicts the photochromic behavior using a drawing of a cartoon turtle. Molecule 4 was also photochromic, but the property was better observed after the product had been recrystallized. When using the laser pointer to draw on these crushed crystals not even a complete spiral could be drawn before the outside of the spiral began to fade (figure 4). The lifetime of the trans-keto form was apparently not as long as with molecule 1.

Figure 3. Molecule 1 selectively irradiated using a blue laser pointer (405 nm) to produce a complicated image.

Figure 4. Molecule 4 selectively irradiated using a blue laser pointer (405 nm) to produce a spiral, illustrating the short lifetime of the red trans-keto form.
Molecules 2 and 3 were bright yellow crystals that fluoresced a bright green upon irradiation with the 405nm light. Although no photochromism was observed, the solid-state fluorescence of these molecules was studied further. Both were examined using a fluorimeter to measure maximum emission and excitation wavelengths, shown in figures 5 and 6 below. Molecule 2 emitted most efficiently at 559 nm and molecule 3 showed a maximum emission at 545 nm. Images of the fluorescence of molecules 2 and 3 were captured and this magnificent fluorescence is shown in figures 7 and 8.
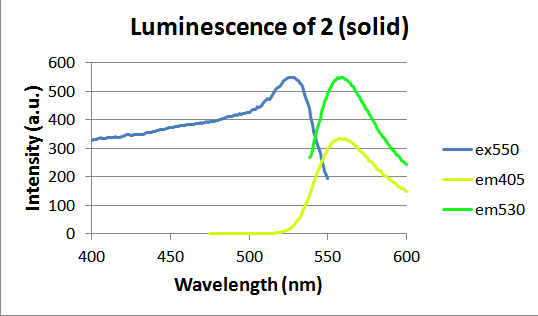
Figure 5. Luminescence of solid molecule 2, where “ex550” represents the excitation spectrum for a 550 nm emission and “em405” represents the emission spectrum for a 405 nm excitation.
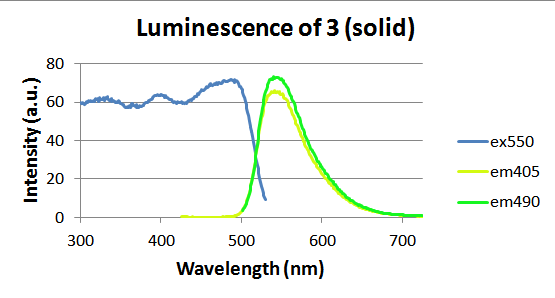
Figure 6. Luminescence of solid molecule 3, where “ex550” represents the excitation spectrum for a 550 nm emission and “em405” represents the emission spectrum for a 405 nm excitation.
The properties of these molecules would be interesting for discussion in a high school setting due to their easy manipulation and as an introduction to organic chemistry. While students may not be able to understand the complex nature of the proton shift that causes the enol to trans-keto transformation, there are several state and national standards that this series of experiments would cover. The execution of the experiment would depend on the maturity and curiosity of the students. For some classes they would be able to conduct the procedure of making the crystals themselves, others would only be capable of manipulating the crystals to observe the properties that they exhibit. If the laboratory only had one fume hood this experiment would prove more difficult to do with a large class. However students could work in groups to examine one crystalline product while other groups research the materials and the properties that the products have. Then they could discuss their finding and how their group’s product reacted to the light.
One such Virginia SOL that could be covered (CH:1) states : “The student will investigate and understand that experiments in which variables are measured, analyzed, and evaluated produce observations and verifiable data.” Selected key concepts include (b) safe use of chemicals and equipment and (d) manipulation of multiple variables, using repeated trials. Safety is a key issue in this experiment due to the toxicity of anilines, and students would have to work in a fume hood, wear goggles, and wear gloves when handling these products. Students will have to manipulate multiple products in order to understand the complexity of the crystals and that different variables will produce different results. Alternatively, in a group scenario students would be expected to produce observations of their product to be shared with the network of other groups.
Inclusion of the salicylideneanilines in a high school curriculum also satisfies objectives of the national standards.3 Some national standards that this experiment would cover include:
- Elements may be bonded together into molecules or crystalline solids.
- The physical properties of compounds reflect the nature of the interactions among its molecules. These interactions are determined by the structure of the molecule, including constituent atoms and the distances and angles between them.
- Light can initiate many chemical reactions.
- In other reactions, chemical bonds are broken by heat or light
- Carbon atoms can bond to one another in chains, rings, and branching networks to form a variety of structures.
All of the products formed crystalline solids, and the properties and the structure of the anilines involved in the reaction highly influences the properties of the product. It is important for students to understand that chemical reactions can take place without the addition of other chemicals or elements to the system, which in directly observable in this isomerization. This experiment introduces them to the network of carbon atoms to form structures in large molecules. Also it shows students that light can be used to break bonds and cause chemical changes or reactions within molecules.
Conclusion
While several anilines were combined with salicyladehyde during the course of this study, only two molecules were found to be photochromic. One of them has hydrogen while the other has an ether group branching off the carbon ring, and therefore it is difficult to know before the reaction takes place if specific anilines will cause the photochromic property. More tests are needed to find other salicylideneanilines that exhibit photochromism. Additionally, it will be interesting to study the length of time that the trans-keto form will stay before reverting back to the molecule’s original enol form (and color). This further research would help in the development of the lab for the classroom so that students would have multiple molecules to analyze. Due to the complex and interesting nature of the photochromism, students would benefit in participation in this proposed lab because it would be enjoyable to analyze the molecules, and would introduce them to organic chemistry, which is often lacking in a high school setting.
References
(1) Cohen, M.D.; Schmidt G.M.J. “Photochromy and thermochromy of anils.” J. Phys. Chem 1962, 66, 2442-45.
(2) Harada, J.; Fujiwara, T.; Ogawa, K. “Crucial role of fluorescence in solid-state thermochromism of salicylideneanilines.” J. Am. Chem. Soc. 2007, 129, 16216-16221.
(3) National Science Education Standards. (2001) National academy press: Washington. 179.